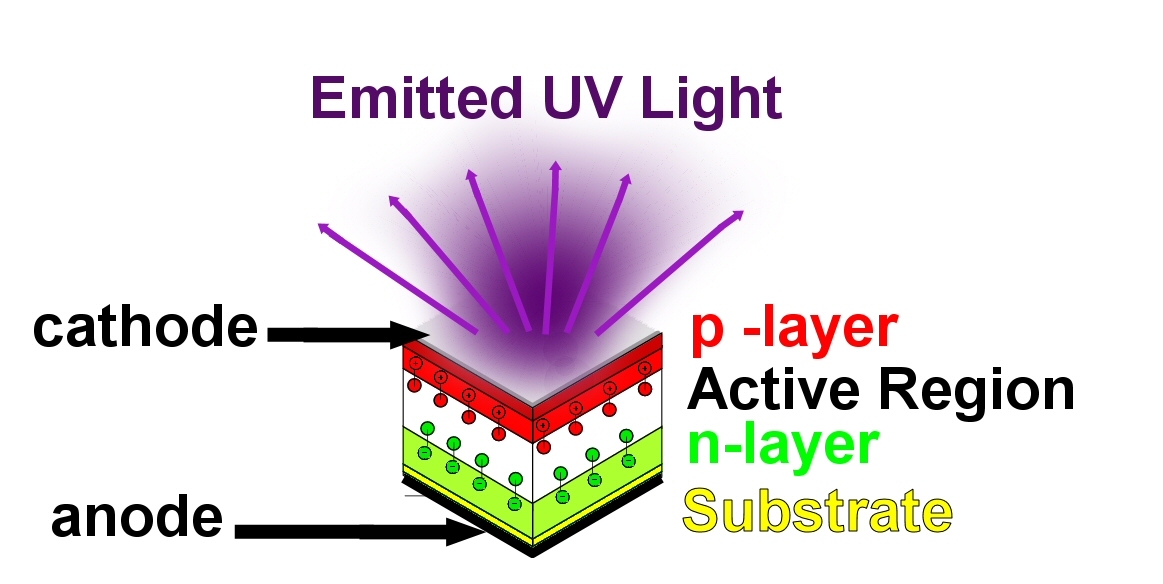
A pictorial example of an LED’s construction is shown in Figure 1: LED Construction. Simply put, an LED is a solid-state device that produces light when an electrical current is allowed to flow from the positive (anode) side of the circuit to the negative (cathode) side.
Taking a deeper look, the internal workings of the LED is Figure 2: LED Electrical Flow
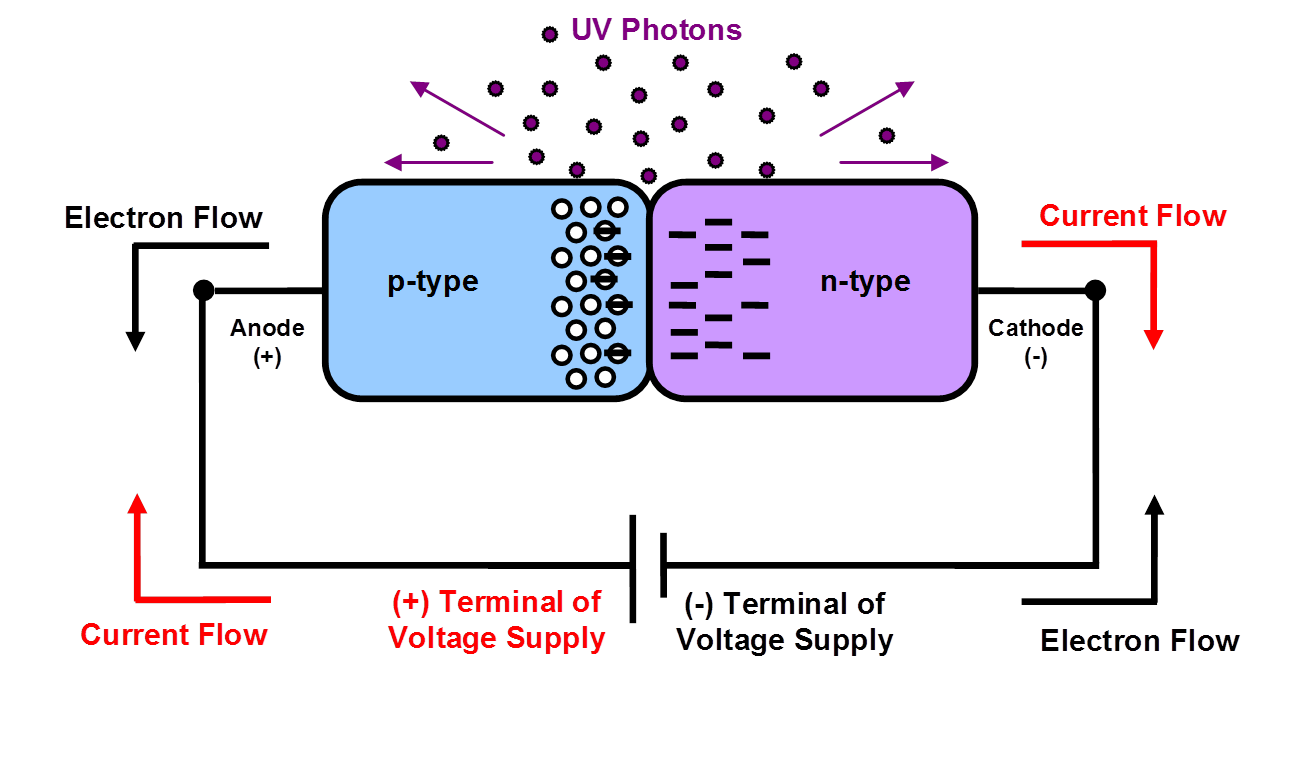
An LED is simple a P-N junction (positive-negative junction) semiconductor where electrons flow from the N-type Cathode across the band gap to the P-type Anode side, while current flows in the opposite direction. When electrons enter the P-type a photon is emitted, i.e. light. A P-N junction is basically one-half of a transistor that we are all familiar with in computers and electronics. Thus they share many of the benefits of transistors.
Diode manufacturers have many decisions to make when developing an LED, from thickness of material to type and density of components. Each decision has an impact on the wavelength, power, and longevity of the LED. LEDs today can emit light across many wavelength ranges, from infrared (700nm+), to visible (390nm-700nm), and ultraviolet (235-390nm). There is even exploratory work on LEDs operating below 235nm.
What is not shown is the fact that any energy that does not emit from the LED as light ends up as heat. So if an LED emits 10% of its energy as light then 90% is converted to heat and must be dealt with. That is the topic of the next newsletter article. Stay tuned.
